

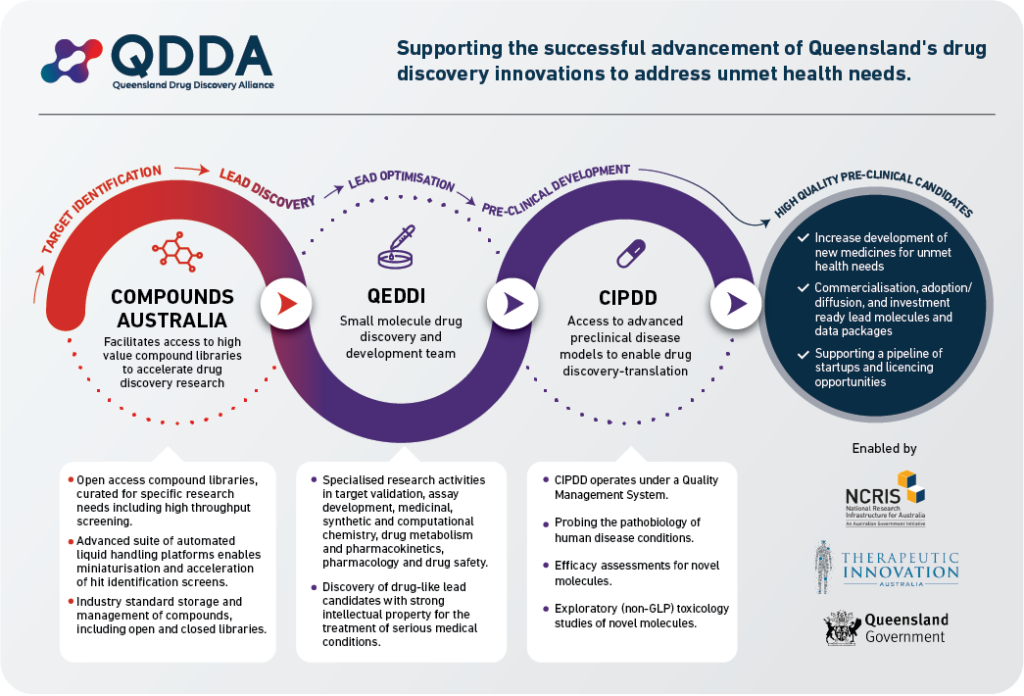
Therapeutic Innovation Australia (TIA) has brought together three of its Queensland-based small molecule translational research facilities to support a vibrant pipeline of novel medicines. Our aim is to streamline enhanced and interconnected access by academic and industry groups across TIA’s network of drug discovery capabilities and expertise, bridging the early stages of development. The three facilities are Compounds Australia at Griffith University’s Institute for Biomedicine and Glycomics, the Queensland Emory Drug Discovery Initiative (QEDDI), a business unit of UniQuest, and the Centre for Integrated Preclinical Drug Development (CIPDD) at the School of Biomedical Sciences at the University of Queensland. These facilities are financially supported by the Commonwealth and Queensland Governments through the National Collaborative Research Infrastructure Strategy (NCRIS) and the Research Infrastructure Co-investment Fund (RICF), respectively, with additional support from Queensland Health and host institutions.






Compounds Australia provides compound libraries and compound management services to Queensland and Australian researchers. Based around a custom-designed suite of advanced robotics and high-density compound storage, Compounds Australia accelerates drug discovery by facilitating access to screening libraries and providing those libraries in customised assay-ready microplates ready for screening.
QEDDI, a business unit of UniQuest, the commercialisation company for the University of Queensland, specialises in translating academic biomedical research into drug candidates for partnering. QEDDI provides expert services in target validation, assay development, synthetic, medicinal, and computational chemistry, pharmacokinetics, drug pharmacology, and safety assessments, creating investment-ready lead molecules and comprehensive data packages aligned with industry and regulatory standards.
CIPDD provides advanced preclinical disease models and services for drug discovery-translation, enabling researchers to develop novel disease models, explore human pathobiology, and screen new compounds for in-vivo therapeutic efficacy, under a robust Quality Management System that meets international regulatory requirements.
Thank you to everyone who attended our Virtual Roadshow on March 18th 2025! If you missed it, catch up now to see how researchers from both the public and private sectors can access QDDA facilities, including Compounds Australia, the Queensland Emory Drug Discovery Initiative (QEDDI), and the Centre for Integrated Preclinical Drug Development (CIPDD).